Are there any acid reflux drug lawsuits?
Feb 25, 2022 · Free Consultation With a Proton Pump Inhibitor Lawyer Nexium, Prilosec and other acid reflux drug side effects may increase risk of kidney injury. Lawsuits reviewed. Review A Case Related Stories...
Can prescription drugs lead to a lawsuit?
After learning about the voluntary Zantac recall and it’s generic form, Ranitidine, former Cleveland Police Officer Michael Konn hired local firm, Kelley and Ferraro. He is now one of a growing...
Is there a lawsuit against Nexium?
Feb 22, 2018 · Acid Reflux Drugs Risk Of Cancer. A recent article from Science Alert has revealed a troubling finding: some medications that have regularly been used to treat heartburn and acid reflux may be linked to stomach cancer. In fact, the risk may be more than double the average risk, according to new research.
Can I get compensation for kidney problems caused by acid reflux drugs?
Mar 18, 2016 · The product liability lawyers at Saiontz & Kirk, P.A. are now investigating potential Nexium lawsuits, Prilosec lawsuits and other acid reflux drug lawsuits for users of popular proton pump inhibitors (PPI) who have been diagnosed with: Acute Kidney Injury or Interstitial Nephritis. Chronic Kidney Disease.
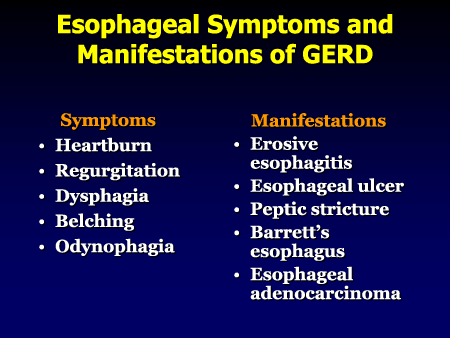
How much is the average Zantac settlement?
about $300,000Zantac Bladder Cancer Lawsuit Settlement Amounts The average settlement in that class action bladder cancer lawsuit was about $300,000.Mar 28, 2022
Is there a class action lawsuit against omeprazole?
Is there a lawsuit against Prilosec? Yes. 1000s of claimants have launched personal injury claims against the makers of Prilosec in the last few years. These cases have been consolidated into an MDL, which should help speed up the process in the courts.
Is there a lawsuit against pantoprazole?
Pfizer to pay $55 million in Protonix lawsuit settlement over claims Wyeth illegally promoted drug for unapproved uses. The world's largest drug maker, Pfizer Inc., will pay $55 million plus interest to settle charges by the U.S. Department of Justice that the acid reflux drug Protonix was promoted for unapproved uses.Feb 4, 2013
Which heartburn medication has a lawsuit?
A Zantac lawsuit is a legal claim filed by people who took Zantac and ranitidine contaminated with NDMA and later developed cancer. People filing Zantac lawsuits are seeking compensation from the drug's manufacturers for bladder, stomach, esophageal, liver and pancreatic cancer potentially caused by NDMA exposure.
What are the risks of long-term use of omeprazole?
Prilosec (omeprazole) is a proton pump inhibitor that treats severe stomach acid-related conditions like GERD. Common Prilosec side effects include headache, stomach pain and nausea. Long-term Prilosec use has been linked to kidney damage, bone fractures and other dangerous side effects.
What are the effects of long-term use of omeprazole?
Long-term side effects Taking omeprazole for more than a year may increase your chances of certain side effects, including: bone fractures. gut infections. vitamin B12 deficiency – symptoms include feeling very tired, a sore and red tongue, mouth ulcers and pins and needles.
What are the long term effects of pantoprazole?
Taking pantoprazole long-term may cause you to develop stomach growths called fundic gland polyps. Talk with your doctor about this risk. If you use pantoprazole for longer than 3 years, you could develop a vitamin B-12 deficiency. Talk to your doctor about how to manage this condition if you develop it.
Does pantoprazole affect your liver?
Pantoprazole therapy is associated with a low rate of transient and asymptomatic serum aminotransferase elevations and is a rare cause of clinically apparent liver injury.Apr 15, 2019
Is pantoprazole hard on the kidneys?
However, there have been few reports of kidney damage associated with pantoprazole. Early and correct diagnosis of pantoprazole-induced acute kidney injury may be the key to treatment. The right diagnosis and early treatment are closely associated with improved prognosis.Apr 23, 2018
Is it too late to file for a Zantac lawsuit?
Zantac Cancer Lawsuits in 2021 – It's Not Too Late to File.
How can I prove I took Zantac?
Proving use of over-the-counter Zantac is a little more difficult, but can be accomplished through receipts, notes in your medical records or even basic corroborating evidence or statements from the people you live with that support the fact that you regularly took Zantac.Jan 10, 2020
Who qualifies for Zantac lawsuit?
You must have purchased Zantac from a pharmacy or other drug store. You must have relied on faulty warnings that did not specify that NDMA or other component parts within Zantac may lead to health problems like cancer for you or a loved one after taking Zantac.Mar 19, 2021
What happens if a drug is not safe?
If it is discovered that a drug is not safe, for example, the drug causes serious side effects-the company may be liable for damages.
How to contact Parker Waichman?
We protect your interests throughout your claim’s process. To schedule your free consultation, call 1-800-YOURLAWYER (1-800-968-7529).
What is lost wages?
Lost wages, if the victim missed work to recover from his or her injuries. The cost of any future medical care that may be required, such as physical therapy or surgeries. The cost of altering a residence, such as installing wheelchair ramps or chair lifts. Pain and suffering.
Does Helicobacter pylori cause stomach cancer?
This bacterium lives in the bodies of more than half of the humans on earth-often with no complications. However, in a small fraction of these individuals, the bacterium has been linked to developing stomach cancer. According to previous studies, those who have lingering Helicobacter pylori infections and take PPIs have a higher risk ...
Can PPI cause stomach cancer?
At this time, it is not understood how the medications and the bacteria possibly triggered the development of stomach cancer. Previously, researchers believed that ridding the body of the infection before using PPIs could reduce the risk of cancer. However, recent findings show that this may not be true.
Is PPI safe for long term use?
However, unnecessary long-term use should be avoided.”. Wong and his colleagues analyzed a Hong Kong database.
Can acid reflux cause cancer?
Acid Reflux Drugs Linked to Stomach Cancer. Acid Reflux Drugs Risk Of Cancer. A recent article from Science Alert has revealed a troubling finding: some medications that have regularly been used to treat heartburn and acid reflux may be linked to stomach cancer. In fact, the risk may be more than double the average risk, according to new research.
Defective and Dangerous Medication Lawsuits
Drug manufacturers have a duty to make sure that medications they sell are not unreasonably dangerous and contain sufficient warnings about dangerous side effects so that physicians and patients can evaluate the risks and benefits before taking the medication.
Previously Investigated Drugs
The drug injury lawyers at Saiontz & Kirk, P.A. previously reviewed and investigated potential claims for users of the following medications. New cases are no longer being accepted for users of these medications at this time, but the following pages are available for informational purposes.

Popular Posts:
- 1. how long does it take to become a lawyer in the philippines
- 2. why did bill cosby fire lawyer?
- 3. how much can i make as a lawyer
- 4. can a lawyer who forms a corporation be the incorporator quizlet
- 5. how do i file a petition for custody without a lawyer?
- 6. the movie where the lawyer quits after winning -weinstein
- 7. what types of classes should an aspiring entertainment lawyer take
- 8. how would you address a doctor-husband and a lawyer-wife
- 9. how much does a corporate lawyer earn in usa
- 10. the girl who played on jag lawyer